Geoff’s Narrations
The GIST
The Blog
Tantalizing, exhilarating, frustrating, hopeful – Solve M.E.’s recent “Immune Dysfunction & T-Cell Exhaustion via Single Cell Immune Profiling in ME/CFS & Long COVID” webinar was all of these things. Researchers have shied away from ME/CFS for decades, and National Institutes of Health (NIH) funding is still in the pits, but what I came away with from this webinar was a sense, once again, researchers are missing out by not tackling ME/CFS.
Don’t they know they’re missing the opportunity to blow the doors open on not just one but possibly many diseases? Apparently not. I have the feeling there will be some teeth gnashing at some point over paths not taken.
To be sure, T-cell exhaustion is one area the NIH didn’t miss. Kudos to them for giving Liisa Selin a major RO1 NIH grant to study it.
It’s always good to go back and see how something like that happened. Not surprisingly, it started with a small patient-funded grant (your dollars at work). Solve M.E.’s 2020 Ramsey award helped Liisa Selin and Anna Gil gather the data needed to score that $2.5 million big NIH grant in 2021. Then in 2023, the Patient-Led Research Collaborative, which knows a good thing when it sees it, kicked in more funding for Selin, and then in 2024, it added funding for Roshan Kumar, Anna Gil, and Selin.

Selin and Gil approached Rivka Solomon in 2019, stating they had found some unique immune abnormalities in ME/CFS.
Long-time ME/CFS and long-COVID advocate – and Patient Representative to the two labs working on this research – Rivka Solomon explained that Liisa Selin and Anna Gil told her at the 2019 Mass ME event that their two-woman immunology lab at the UMassChan Medical School, had, while researching EBV, uncovered some striking immune abnormalities in ME/CFS. By that time, Liisa Selin, an accomplished researcher, had been living with ME/CFS since 1975. In order to keep from being ostracized, she’d laid low about her ME/CFS for decades.
The Hypothesis
Their hypothesis—that a dysfunctional response to an immunological trigger (infection) results in a chronically hyperactive immune response—is not novel. It’s the last part—the T-cell exhaustion—that is so tantalizing because, as we’ll see, their T-cell work provides the potential for getting at the actual starting place—when these diseases began.
The work is in good hands. If anyone is a T-cell expert, it’s Liisa Selin: she’s co-authored almost 60 papers on T-cells over the past 35 years.
The Gist
- Tantalizing, exhilarating, frustrating, hopeful – Solve M.E.’s recent “Immune Dysfunction & T-Cell Exhaustion via Single Cell Immune Profiling in ME/CFS & Long COVID” webinar was all of these things.
- It was exhilarating because Liisa Selin, Ana Gil, and Roshan Kumar believe they have a shot at figuring out what, at the immune level, started things off for ME/CFS and long COVID. It was frustrating because despite their progress, they lack the funding to get there right now.
- The work got going with a 2020 Solve ME Ramsey Award grant which laid the foundation for a big 2021 NIH grant, and has been supported since then by two Patient Lead Research Foundation grants in 2023 and 2024.
- Their hypothesis—that a dysfunctional response to an immunological trigger (infection) results in a chronically hyperactive immune response—is not novel. What’s so exciting is their T-cell exhaustion work, led by long-time T-cell researcher Liisa Selin (a long-time ME/CFS patient).
- Talk about exhaustion. They’ve found that the T-cells in ME/CFS and long COVID patients are producing very little of the substances (perforin, granzymes) they use to kill virally infected cells, and very few of the cytokines (IFN-y, TNF-a) needed to orchestrate the immune response.
- That means that one of the two main players in the adaptive immune response is essentially dead in the water. The cytotoxic T-cells appear to be so messed up that they’ve resorted to producing a strange, rarely seen kind of hybrid T-cell called a double-positive T-cell in great numbers in these diseases.
- In an attempt to get the T-cells moving again, these cells produce high levels of a cytokine called IL-9, which also increases mast cell production and the risk of an autoreactive response.
- Rosh Kumar, the head of research at HiFiBiO Therapeutics, joined the Selin/Gil team in 2024 in the Patient Lead Research Foundation Grant. (Kumar’s wife has had ME/CFS for 20 years.)
- The grant allowed them to use single-cell analyses to dig deep into the gene expression, T receptor repertoires, and T-cell subsets in the cytotoxic T-cells. The goal is to uncover T-cell biomarkers and ultimately—and here’s where it gets really interesting—determine what antigen (pathogen, autoimmune factor) is keeping the T-cells and the immune system ME/CFS and long COVID up at night and driving it (and us) to exhaustion.
- Four hundred thousand cytotoxic T-cells from 16 participants later, they’ve uncovered more intriguing issues. A “quite unusual” pattern of both inflammatory and exhaustion markers is present plus two more types of unusual T-cells popped up.
- Assessing the very, (very) complex cytotoxic T-cell receptor repertoires brought some welcome news. Because the T-cell receptors can be configured to identify virtually any antigen (danger signal) they provide a memory bank of all the immune insults a person has been subjected to. They’re very complex keys which, if researchers can decipher them, could unlock what started these diseases and is keeping them going.
- That process has begun. They were able to identify several T-cell receptor clusters unique to ME/CFS and long-COVID patients that appeared associated with some sort of antigenic driver.
- Getting to the “answer,” i.e., actually identifying the actual culprit(s); i.e. the pathogen and immune dysregulation present, is not going to be easy. For one, while it has long been clear that these diseases are very heterogeneous, it was still remarkable to see a fine-tuned analysis of a very specific part of the immune system (CD8 T-cells) in a small group of patients uncover substantial heterogeneity.
- Even within the double-positive T-cells, they can identify distinct subsets, clonotypes (T-cells with a similar origin), and enriched sequence motifs (which can help them uncover the triggering factor).
- Liisa Selin explained that this makes sense given the different pathogens we are exposed to and the different immune systems we have. Indeed, their ability to identify subsets at the molecular level is part of what makes their work so tantalizing.
- Because T-cell exhaustion has been almost totally explored at the tumor level by uncovering T-cell exhaustion at the global or systemic level Selin, Gil, and Kumar’s work is, in effect, creating a new field of T-cell exhaustion. one that Selin believes could help explain ME/CFS, long COVID, and other diseases.
- Ultimately, these researchers want to uncover biomarkers corresponding to the different molecular subsets and produce tests that doctors can use in the clinic. That’s a big task, but Kumar reported that with the technology available today, it is eminently doable.
- The only thing stopping them now is more funding.
Really Exhausted

Perforin production in unstimulated (left) and stimulated (right) T-cells. It’s easy to find the healthy controls (tall gray bars).
Do you feel exhausted? You are not alone. Your T-cells are probably just as exhausted.
Every once in a while, you see a graph that makes your jaws drop open, and the perforin graph was one. Perforin is the substance T and NK cells use to drill into an infected cell and kill it. Selin and Gill’s studies suggest that ME/CFS and long-COVID patients’ T cells produce almost no perforin…
They’re also producing 4-5 times lower amounts of IFN-y, a central orchestrator of the immune response. Low IFN-y levels alone could result in difficulty clearing pathogens, produce inflammation, impair T-cell metabolism, and promote T-cell exhaustion.
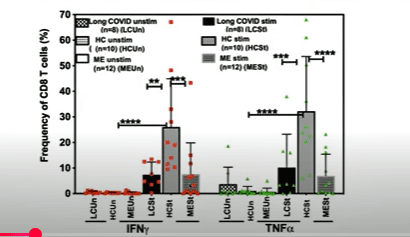
Greatly reduced IFN-y and TNF-a levels in the ME/CFS and long-COVID patients compared to healthy controls (light grey).
Strange T-cells Pop Up
Plus, Selin and Gill uncovered something brand new: a huge increase in a distinct type of T-cell called double-positive T-cell (CD4+ CD8+) in people with ME/CFS and/or long COVID. Instead of being differentiated into the two usual major T-cell subsets CD4 (helper/regulatory) and CD8 (killer) T-cells, these hybrid T-cells look like both CD4 and CD8 T-cells at the same time.
Selin found that these cells increased 8-9 fold in ME/CFS and long-COVID patients. We want dramatic findings like that. We don’t want subtle findings, we don’t want findings that researchers have to do a double pirouette to fit into their hypothesis—we want major differences that stand out like a sore thumb—and that’s what Selin found.
We also want findings that align with symptoms, and that’s what we got as well: the double-positive T-cells were highly correlated with symptoms, but in a strange way. Instead of hurting things, they appeared to result from an attempt to rebalance the immune system. A positive correlation between double-positive T-cell levels and perforin production (more double-positive T-cells equaled increased perforin production) suggested these odd cells were both: a) intimately tied to the reduced T cell cytotoxicity; and b) might be functioning in a compensatory manner.
On the other hand, a correlation analysis suggested that the compensatory response was not, as so often seems to happen with compensatory responses, going so well. The few double-positive T-cells in the healthy controls existed within a tightly integrated system, but the loose correlations found in the ME/CFS and long-COVID patients suggested that a “highly dysregulated” cytotoxic T-cell immune response was present. That suggested that the immune system was kind of flailing around, trying to come up with an answer.

Tightly integrated immune response by healthy controls (left); loosely integrated immune response (ME/CFS/long COVID).
It’s perhaps no surprise that men and women tended to be different – but similar as well. Women produced more of the double-positive T-cells, which produced IL-9. The IL-9 connection seemed particularly apt as it drives T-cell proliferation, prevents apoptosis, and enhances the killing ability of CD8 T cells. Thus, it may be a beneficial compensation acting to help retain cytotoxic function in CD8 T cells.
The Model

The grand model.
In all, if I got it right, the hypothesis goes something like this – an infection triggers a chronically activated exhausted immune response which either fails to clear the infection, reactivates persistent viruses (herpesviruses) or produces an autoimmune/autoreactive response. Our now-wired and tired immune systems try to compensate by producing scads of double-positive T-cells (as well as other unusual types of T-cells).
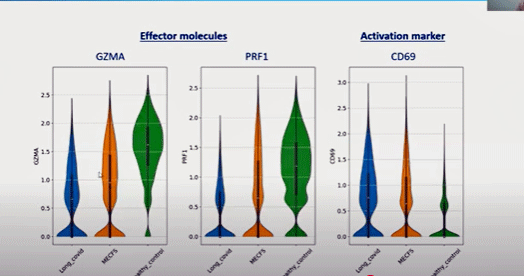
High activation (right box) paired with low effector molecule production (granzymes/perforin—left boxes) in the ME/CFS/long-COVID patients (orange/blue) spells wired and tired cytotoxic T-cells.
Double-positive T-cells produce IL-9, which enhances T-cell proliferation, increases mast cell production, and increases the risk of an autoreactive response. Chronic immune activation alters our metabolism, resulting in increased oxidative stress and lactic acid. Brain fog and autonomic nervous system instability result.
In this scenario, T-cell exhaustion comes first, ultimately resulting in B-cell dysregulation. (Note that Nath believes B-cell dysregulation comes first.) The authors did not explain how T-cell dysregulation could throw the B cells off, but several scenarios exist. Reduced cytokine production could prevent the B-cells from getting on track when pathogens present themselves. Exhausted T cells also produce a factor called PD-1, which inhibits B-cell proliferation and antibody production.
The dramatic reduction in cytotoxic killing ability, the equally dramatic increase in the levels of the strange T-cells, and the high IL-9 levels that may be driving T-proliferation (and exhaustion) were all pretty exciting stuff. The deeper Selin and Gil dug, the more they seemed to find.
It was the next step, though, that was really eye-opening.
Getting at the Source?

Roshan Kumar’s wife has had ME/CFS for 20 years.
Roshan Kumar, PhD, has a special connection to ME/CFS—his wife has had it for 20 years. Kumar is the head of research at HiFiBiO Therapeutics, which develops immunotherapies. He joined the show when he, Selin, and Gill received a PLRC grant in 2024, which funded them to assess the gene expression and receptor repertoires of T cells in ME/CFS and long-COVID patients using single-cell analyses.
The goal is to uncover T-cell biomarkers, and ultimately, what antigen (pathogen, autoimmune factor) might be keeping the T-cells in ME/CFS and long COVID up at night and driving them to exhaustion. They also want to learn more about those mysterious double-positive T-cells that showed up.
Four hundred thousand CD8 and double-positive T-cells from six ME/CFS patients, six long-COVID patients, and four healthy controls later, they uncovered a new suite of issues.
For one, a “quite unusual” pattern of both inflammatory and exhaustion markers was present. Plus two more types of unusual T-cells popped up in the ME/CFS/long-COVID patients. The strange cytotoxic T cells that resembled CD4 memory cells and naïve-like T-cells with distinct expression profiles provided more signs of dysregulation within the cytotoxic T-cell subset.
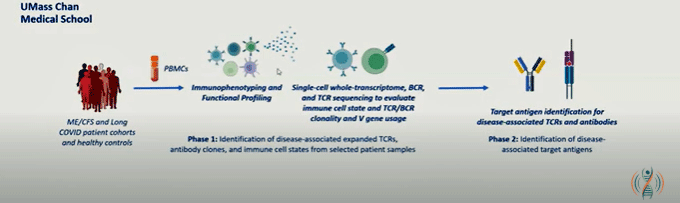
The deep dive: exploring the gene expression of T-cell subsets and T-cell receptors in 400,000 T-cells from each patient.
Next, they looked for commonalities in sequences of the receptors on the exhausted T-cells. Because T-cell receptors are looking for anything that can tweak the body, they’re amazingly complex, and trillions of possible combinations of T-cell receptors exist. These receptors sitting on the outside of the T-cells provide a memory bank of what the cells have been exposed to. They’re very complex keys that, if researchers can decipher them, could unlock what started these diseases and keep them going.
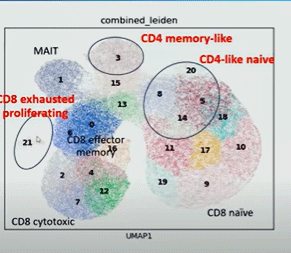
More strange types of cytotoxic T-cells show up.
Focusing on the gene sequences that deal specifically with antigen recognition, they were able to identify several clusters of similar T-cell receptors unique to the ME/CFS and long-COVID patients. Those clusters suggested that the receptors in the exhausted cytotoxic T-cells in the ME/CFS and long-COVID patients were responding to a common antigen; i.e. the team had made a “first step” in identifying what is turning on these T-cells – and perhaps what turned on these diseases.
Getting to the “answer,” i.e., actually identifying the actual culprit(s), is not going to be easy – but it is doable. For one, while it has long been clear that these diseases are very heterogeneous, it was still remarkable to see a fine-tuned analysis of a very specific part of the immune system (CD8 T-cells) in a small group of patients uncover substantial heterogeneity.
Even within the double-positive T-cells, they can identify distinct subsets, clonotypes (T-cells with a similar origin), and enriched sequence motifs (which can help them uncover the triggering factor).
Selin noted that the heterogeneity makes sense. We are all, after all, exposed to different pathogens, and all have different immune systems. Indeed, their ability to identify subsets at the molecular level is part of what makes their work so tantalizing. To get at these diseases, we need molecular signatures that can tease out the subsets and point to treatments. Once that happens, Kumar said, many immune modulators are available.
On that note, Kumar, whose company develops checkpoint inhibitors, feels we need to understand more about the immune system before using them. You wouldn’t want, for instance, to stimulate autoreactive T-cells and cause more inflammation.

Disentangling what’s happening with the T-cell receptors could be the key to understanding the immune aspects of ME/CFS, long COVID, and perhaps the whole diseases.
No standard blood tests are currently available that patients can get to assess T-cell exhaustion. Even in the lab, researchers usually only assess T-cell exhaustion within tumors, which is how checkpoint inhibitors came about. Until ME/CFS and long COVID, no one had been looking at “global” or systemic T-cell exhaustion. Selin, Gil, and Kumar’s work is, in effect, creating a new field of T-cell exhaustion – one that Selin believes could help explain ME/CFS, long COVID, and other diseases.
Ultimately, these researchers want to uncover biomarkers corresponding to the different molecular subsets and produce tests that doctors can use in the clinic.
Many studies are helping us understand the molecular problems in these illnesses, but what makes this work enticing is its potential to dig into the T-cells—”the sentinels of the immune system,” as Kumar called them—and possibly get at the prime mover of these illnesses.
Kumar says that it’s entirely possible to do that with the technology available now. The PLRC grant did what patient organizations do best—it allowed them to start down this path. The only thing stopping them now is more funding.
- Up next for Solve ME’s Webinar Series – none other than Akiko Iwasaki on “Probing Functional Antibodies in Patients with ME/CFS“





“That suggested that the immune system was kind of flailing around, trying to come up with an answer.”
A lot of hard hard science in the blog, but I have some soft science: The few occasions I feel noticeable improvement take place immediately after I’m sick with a virus (when my immune system, I guess, hasn’t been “flailing”).
Furthermore, the only time I can nap without triggering a migraine is when I get a vaccine shot.
My PWME feels the same when sick with a virus. Somewhat better and never gets a migraine when sick. Napping can often result in a migraine as well.
I’ve noticed that too. I have a mini-remission after I get a cold, but I pretty much never get colds.
Ditto .
Have you tried CD38, Daratumabab to give to patients having ME?
Thank you, Cort, for writing about these groundbreaking researchers. I am so lucky to get to work with them.
I hope everyone watches the SolveME webinar of April 29, 2025, to hear more from Liisa Selin MD, PhD, Anna Gil PhD, and Roshan Kumar PhD. You will also get to hear from both me and Megan Fitzgerald, the two Patient Representatives on this exciting research project. Find the webinar here: https://www.youtube.com/watch?v=2DQZp48fyek
** And even more, I hope everyone will check out the Selin Lab website (link here: https://www.umassmed.edu/selinlab/) so they can learn more about our work, and then… yes, then DONATE! **
Your donations allow this dedicated team of researchers to continue with their important work. Let’s find some answers!
Best,
Rivka
Correction: Megan Fitzgerald PhD (can’t forget the PhD!)
Thank you for your work! Please keep at it
Cause? Treatment? Cure?
Classifying us into groups or finding more ways to give us a diagnosis isn’t getting us better. And just used up a lot of research effort and funding.
I’m grateful for the insight, I suppose – and appreciate the information – but I’m 75 and don’t have a lot of years left to enjoy a better life after 35 years and my career in fusion physics lost, and the only thing the psychological theorists are going to respond to is patients who get well and stay that way, so: theories on how to unexhaust the T-cells? Are they still usable if woken up? Does bringing them back to life fix things?
How long before we might have that kind of answers? Are there already drugs which work?
Thanks.
Well put
This might very well be the cause of our symptoms, disregarding this would be a mistake. We also have drugs that can reverse it.
Cause?
My hypothesis resembles the chicken or egg question. Does the immune system fail to kill pathogens because it is exhausted? Or does is need to act as if it were exhausted because it fails too long to deal with the pathogen at hand?
That’s largely a question about onset. If it starts by the immune system being exhausted and therefore failing to kill the pathogen, it will need to keep trying and hence keep being exhausted. If it starts by the immune system failing to kill the pathogen and therefore needs to tone down because too long strong immune activation would cause massive harm to the body, then it will fail to kill the pathogen even more after it is toned down. Both have good chances to give rise to a vicious circle that’s hard to break.
For any innate immune response (in an otherwise healthy person), the first 24 hours display a massive upswing of innate immune reaction and then a rapid decline in innate immune strength *in the hopes the body starts to find a proper antibody*. That often won’t happen within that first 24 hours, but after initially mounting a very strong innate immune response, going a few tones lower is often enough to temporarily keep the pathogen under control until the adaptive immune system finaly kicks in and succeeds.
When failing to find an antibody, the innate immune system has IMO to be stuck in this second stage of keeping the pathogen under control without being able to fight it off completely. Producing copious amounts of ROS, both immune cells and our mitochondria, are part of that phase. The longer it takes, the more moderated the immune strength in this phase has to be in order to not cause too much damage to our own cells including not inducing ROS based cancers. With that, the innate immune system will be even more challenged to keep pathogens under control. And the singalling to the adaptive immune system to keep trying finding good antibodies will be decreased too. That will decrease changes for the body to get unstuck. On a positive note, the adaptive immune system IMO needs to reduce its search for antibodies against pathogens in this prolongued phase as weeks or more of intense and quite unsuccessful searching for antibodies IMO is a very strong recipe for developping a series of auto immune diseases. Itaconate dampens both (first and foremost) the strength of the own immune cells and secondary also of all nearby cells to some extend to. And it happens to reduce development of auto immune diseases a lot.
If the immune system quickly finds antibodies to the pathogen but with a lot of crosstalk / crossreactivity to the own cells, roughly the same needs to happen. The adaptive immune system needs to tone down a few nothces as it sort of has proven it does a rather brute force job causing plenty of damage on the side. And the innate immune system including the mitochondria going into defensive mode needs to take up the slack to try and do nothing but hibernate / Dauer until the body hopefully finds a way out. Control the spraid and multiplication of pathogens and survive until the body knows how to kill it without killing or badely damaging the host.
That pattern fits a lot with harsh infections like adult EBV infection or a rather new pathogen popping up like Covid. It also fits the observation that many people with long Covid weren’t that sick from Covid itself. Remember: in Covid much of the symptoms and casualities are not from the virus itself, but from the rather strong reaction of the immune system to the virus. Relating this to the above hypothesis: IMO for many long Covid patients the body quickly decided it was too risky to go long term full innate immune activation in an attempt to strongly fight the virus in the first phase and push the adaptive immune system to push out plenty and plenty attempts for good antibodies. That, so long innate immune activation is strong enough to keep viral load under control enough, avoids the risk for very strong immune activation that killed many patients and rendered many other patients’ lungs that healed to a fibrosis infested organ (many Covid lungs are 50% fibrosis tissue). But the other side of the coin is that the body missed the opportunity to find good working antibodies as it had a lower setting on “try to find good antibodies at the risk of inducing auto immunity”.
Here again we see a common pattern in ME/CFS and most likely long Covid: lack of observable damage in those with long Covid compared to plenty of damage in many Covid patients who had a strong accute version of it. That easily misleads doctors into thinking “if I see so much damage in actual Covid patients and so few in long Covid patients, then the latter can’t be ill at all let alone for so long so badely”. They IMO make the mistake by thinking the amount of damage they observe (acute case) or don’t observe (long case) is directly proportional to the amount of (peak) virus during infection. It is demonstrated that much of the observed damage in Covid is due to the own immune reaction trying to fight the virus more so then the virus itself.
Even if the virus is finally cleared if the body choses the “last it out” method of gradually trying to better control the infection and outlast it, it can easily stay locked in this situation simply because it left the body weeks in a state of very unbalanced immune activity. That gave plenty of opportunistic and partialy commensual microbes the chance to party. And with them partying, now the innate immune system has to keep toning down (keep stuck in Dauer) because activating all of the immune system to keep those (in healthy people harmless microbes) *all at once* (in their unbalanced amounts and aggressive behavior) in check is likely going to cause massive damage just ast well.
The problem with that hypothetical phase is: regular tests will show up nothing, since testing for having antibodies or not to normally harmless pathogens and commensual microbes is useless. Everybody has such microbes living in masses in the many microbiomes in and around their body. So again, what (makes no use testing) is not tested will not show up. And doctors again will find no abnormalities.
The above hypothesis also fits with Brian Buckbee’s and many other patients observations. They feel suddenly “nearly well” like one day before being struck by an infection. My explanation: a new pathogen is reckognized as something new and urgent. That differs from what the body by now knows is long time present and it could keep for a long time already under control by the method of inhibition / Dauer. This new pathogen could be anything with any risk involved. At least it needs to mount a strong initial spike in innate immune response and start trying to make antibodies again even if only for a short period. These two actions require the body to uninhibit these parts of the immune system. Since itaconate (and other stuff) inhibits both, that needs to be downregulated at least for a short period too. And bang, patients have a lot more energy for one day.
Working on the above but in a much longer and better backed by science text. Slowly as it’s a hell of a job and I have a serious case of ME/CFS plus FM too.
Fascinating! Keep working 🙂
This is now a prominent hypothesis – adaptive immune system breaks down leaving the innate immune system to step up….but as you say – that can cause too much inflammation – so it has to be toned down as well…..so you get Dauer if I have it right.
The T-cells certainly showed an immune system desperately trying to work things out, with inflammation and exhaustion markers in the double-positive T-cells.
“When failing to find an antibody, the innate immune system has IMO to be stuck in this second stage of keeping the pathogen under control without being able to fight it off completely. Producing copious amounts of ROS, both immune cells and our mitochondria, are part of that phase.”
Well, either the adaptive immune system fails to find a good antibody in time or the adaptive immune system isn’t given enough time to find a good antibody because the body knows it can be dangerous as that requires “to put the connection between innate and adaptive immune system” a long time on high and that risks to have too long too strong innate immunity settings.
Fibrosis is a natural consequence of chronic inflammation (among other causes). In other words: too long too strong inflammation has very high chances to lead to fibrosis. If the innate immune system would be strongly activated for weeks all around the body then you would have to expect fibrosis all around the body and that would be a rather bad thing. Having weeks in a row of peak innate immune activation like what happens at the first hours of a strong infection would IMO simply kill you before the first week has past. Therefore the innate immune system IMO *must* tone down after too long activation. In other words, it must react sluggish to most except the very urgent issues. I can see that easily is recorded as the (innate) immune system being exhausted.
Just a single round of Covid can be devastating to the lung quality of surviving patients.
Half the lung tissue replaced by fibrosis is detrimental to breathing capacity for the rest of the life and irreparable without lung transplant.
As to continuously pumping out new attempts for antibodies in a partly trial-and-error method, I think it’s even more easy to see how that can go wrong. In most cases it wouldn’t go as quickly wrong as excessive innate immune activation.
That (plus the hypothesis above) is IMO also the reason why so many people have food cravings they know are bad to them. What else is a food allergen but a mostly innocent food particle mistakingly identified as a pathogen. So a sudden influx of those *!!!PARTIAL!!!* (reminder: consuming some allergens can kill you so don’t experiment!) allergens gets according to the above idea a brief period that the immune system stops pumping out inhibiting chemicals because the innate immune system (even if “true” allergens are mostly detected via trained immunity) IMO needs to check first the nature and risk of this sudden immune threat. That gets many people a brief and quick boost in feeling afemore energetic, long before for example the chips are digested and providing callories in my case. And after it, the dip comes ;-).
This paper is one providing an overview of the links between immune activation and fibrosis that looks good at first sight. I didn’t take the time to read it in depth.
https://pmc.ncbi.nlm.nih.gov/articles/PMC3292796/
Why doesn’t IVIG help T cell exhaustion?
Good question. I can see two parts:
* Much of the triggers of innate immune cell activation are not dependent on antibodies nor pathogens. Oxidized cholesterol created by too much oxidative stress and damaged DNA from an overactive innate immune system killing some of our own cells can trigger innate immune system cells too. Those activated (at more then resting and less then peak intensity) immune cells in turn can create oxidative stress and damage to our own cells… that in turn… form enough damaged parts to create a self maintaining loop.
It is suspected that many cases of viral induced ME/CFS last long after the initiating virus has been iradicated, like after a bout of flu for example. Similar is suspected in many long Covid cases or at least it is very hard to find any virus left. The viral onset plus circumstances is enough to create a vicious circle. Eliminating the virus that started a case of ME/CFS might reduce how difficult it is to get out of ME/CFS, but insufficently. In many cases the body will already have succeeded doing that on its own, but even if it didn’t yet trying to eliminate it IVIG may be insufficient to break the vicious circle. And as science points out and Issie stressed a lot: IVIG has known side effects too risking to pose a burden on our weakened health that may reduce, nulify or reverse its use to certain patients.
When it comes to comensual microbes turning bad: how many antibodies have healthy donors to those? And would trying to eliminate all comensual microbes turning bad be a good thing? We have microbiomes in many places and good health depends on them. There it’s rather hard to distinguish friend from foe and it often depends on circumstances. Repeativly smashing our microbiomes with a big slashhammer in the hopes to weed out the bad ones and leaving only the good ones sounds rather optimistic to me.
* A second part: if the body decided it somehow needs to curtail the risk of adaptive immunity getting out of control, then it would do (from a system engineer point of view) wise to not inhibit a single parameter (like antibody production) very strongly while leaving other parameters (things like but not limited to how strong immune cells react to antigens binding to antibodies) unchanged. Downregulating many parameters a bit can result equally well in a strong downregulating of the thing the body might want to downregulate, without leaving massive open holes in its defenses. Completely blocking one parameter sort of is the equivallent of throwing a very big spanner in the wheels leaving the system no flexibility for when a new threat emerges. Spreading the risk by adapting plenty of parameters a bit leaves much more options open. That is consistent with ME/CFS. While it seems that ME/CFS patients have massive problems with their immune ssystem, they often seem remarkable resilient in surviving pathogens and other immune threats without succumbing into bad accute illness. If that reasoning is right, likely the body even tries to compensate for whatever we try to do to take away the supposed immune blockage. Many patients have observed that it is almost as if their body actively tries to keep them ill by trying to restore any symptoms within days or less every time we find a promissing fix.
Ah, yes chips….years ago I would eat one single chip and be very,very ill for a week or longer. Starch???
Also, one of my first clues something was wrong was when I would go out and have a couple alchohol drinks, I would be hung over for the same, a week or more
Wow, how are you able to have such a good cognitive ability ? I’m always amazed by what you write , knowledgeable and prolific. How long have u been dealing with m.e /cfs ?
Some people do well with LDN- Low dose Naltroxen
I’m I do not understand this. It just seems backwards to me.
“Classifying us into groups or finding more ways to give us a diagnosis isn’t getting us better.” as this is precisely what I believe needs to happen first!
But, but, but can you get to a treatment in a heterogenous disease without understanding which types of patients are in that disease? (And then are able to target them?) How can you hope to have a drug trial that works when you essentially have a bunch of different diseases in your trial? IMHO subsetting is everything 🙂
Yes, checkpoint inhibitors can turn T-cells on again and have improved survival rates significantly in some cancers – which is why 11 exist. Immunomodulatory drugs can be very helpful as well. Witness what was available to people with rheumatoid arthritis 30 years and today. While not everyone is helped many people are helped tremendously.
To me the key is going to be to find molecularly defined subsets that point arrows right at treatments. When we get to those molecularly defined subsets I am very optimistic that we’ll be able to look out and find some treatments are already available.
There’s no doubt that figuring out what’s turning on the T-cell subsets and how they are affecting different patients is a huge project but for me this seems like one of few efforts that has a plan to get to the bottom of things.
They just need funding.
I definitely agree. And there is little to no discussion I’ve seen on genetics. Part of my recovery came from genetic testing (Genomic Insight not 23&me type) and comparing those findings with suggestions found here and on other ME/CFS pages. For me, methylene blue is not an option. It could kill me due to G6PD gene. Dapsone, High dose Vit C (over 1000mg), Fluoroquinolone, Peppermint oil are contraindicated for the same gene. And that’s just one gene and there are dozens of more genes suggesting things to do or not do for better health. In my case it’s high dose thiamine. You’ll never know until you are tested.
Well said
Low perforin was pinpointed many many years ago.
Is the T Cell exhaustion the by product of immune factors? Or autonomic dysfunction? Or both? I suspect the latter.
To what extent does T cell exhaustion contribute to symptomology, versus being a byproduct of something else (autonomic dysfunction) that is driving symptomology?
Let’s see what Polybio’s antiviral studies show. If the t cell exhaustion is a result of viral reactivation, then antivirals might help.
I am not convinced by viral reactivation. But let’s see
Or maybe antivirals to knock down the virus more with immunomodulators to buck up the immune system. (There’s got to be an immune problem). How about mitochondrial enhancers to give the T-cells more of a boost at the same time? It’s going to be interesting 🙂
The T-cells (and the immune system they exist in) may have some inherent defect that prevents them from clearing out infections – leaving them chronically activated trying to fight off the infection.
Since immune exhaustion seems widespread (NK, B- cells as I remember) it may all come back to problems generating energy. When activated immune cells have to generate a tremendous amount of energy. IF they’re unable to do, that may be why they are they have to keep fighting and fighting.
Selin and company believe the T-cell exhaustion comes first and leads to autonomic nervous problems.
It’s very complex but their studies suggested that markers of Tcell exhaustion were correlated with symptoms; i.e. it does appear to play a role.
Yet the correlation between level of T cell exhaustion and symptom severity does not automatically mean causation. It might – but it also might not.
Hi Cort, and hi everyone.
I would like, up front , to make my central new point, but also harken back to points that I have been bringing to this discussion for a few years.
My new point is:
based on reading Cort’s synthesis of the study: I see a great similarity to cancers. I am in no way suggesting that cfs is based on a cancer. But the key point there is: mutation… OR.. incomplete cell division or cell differentiation or immune cell Training.
Mt brother passed away in 2012 of lymphoma coming from white blood cells that had been released from the bone marrow BEFORE they had been (a( differentiated, (b) trained (c) approved… and he felt that toxicity had been the cause.
If we are discussing hybrid CD4+/CD8+ t-cells, then it suggests one of two conditions:
either (1) the cells had not finished differentiating, possibly due to whatever cause made them exhausted,
or
(2) this is a new wrinkle of normal immune life of which science was not previously aware (or at least concerned)
My older points comprise the following:
-the american society that concerns itself with Sepsis made a statement during the pandemic that ling covid IS sepsis.
-sepsis is a dangerously high toxin level.
If high enough it can result in a hypothermic dauer-like state rather than allow you to die
-The first steps , i think,
for any cfs patient to RECOVER
ARE:
-to detox
-to assess what unsuspected toxicities may have triggered cfs.
Some cite Epstein Barr, s
Yes…I am doing heavy metal detox now and I can tell you its making my immune system flail all over. Inflammation rears its ugly head for a day, then it goes back down, then a bunch of bumps on the back of my neck pop up and go back down, then the acne scaring on my upper back and shoulders will actually produce hundred bumps only to go away the very next day
Question is, is it actually doing any good are simply causing an immune response in different parts of my body. Dreaming again for the first time in many years…but last night bad dreams/nightmares. I’ll keep detoxing
Great article, thanks Cort!
Thanks!
I recently had blood tests done in Germany to ascertain if I may have chronic Lyme. The results were as follows
NK Lymphocytes:
NK cells CD3-/CD16/CD56+ (rel.): + 31.5(+) (ref range 3.0-13.0)
NK cells CD3-/CD16/CD56+ (abs.): 472.0 (ref range 80.0-600.0)
NK-cells CD56+/CD57+ (rel.): 1.8
NK-cells CD56+/CD57+ (abs.): – 27.0(-) (ref range: > 60.0)
Ratio CD57+ NK-cell: 0.06
This seems to be something different to what this article is talking about? The consultant I spoke to said that my killer cells for viruses were actually very good, but for bacteria not good at all, and this implied chronic lyme.
I don’t see any reference to CD8 T-cells.
But like others I resonate with “That suggested that the immune system was kind of flailing around, trying to come up with an answer.” – I sometimes feel my body is fighting something and figuring out what it is and what to fight it with, and eventually decides on a herpes outbreak… wish all of this made sense to me.
It’s too bad you didn’t get CD8 cells analyzed but I was going to add something about NK cells, but I accidentally erased it. Something similar may be going on in both NK and T cells. NK and T-cells derive from the same immune cell lineage and kill virally infected cells the same way.
Cytotoxicity in NK cells in ME/CFS patients is down by about 50% and they were the first immune cells in ME/CFS which researchers thought had become exhausted. It looks like your NK cell cytotoxicity is down – which is common in ME/CFS.
I plugged your numbers into Perplexity AI and asked it what it made of them. It clearly wanted more data – which you may have but this was its first shot.
“These results should be evaluated alongside symptoms and other lab markers. The pattern of elevated immature NK cells with reduced mature cytotoxic NK cells often appears in autoimmune hepatitis, primary sclerosing cholangitis, or systemic autoimmune diseases. Further testing for autoantibodies, liver function, and inflammatory markers (CRP, ESR) would be warranted”
Perplexity AI? Wow, I didn’t know about this! A new resource!
I just got the results of two very exotic tests; T Helper IL-17 and T Cell Interleukin Prolif. Apparently my T-Cells are in normal ranges excepting my NK cells which are elevated. In trying to understand all this, I just read that NK secrete perforin as well and are considered related to T-Cells.
I don’t have detectable IgM and my response to certain kinds of vaccines (like Prevnar) is sub par. Some mornings I wake up and feel almost normal but other times I feel God awful. PEM abounds.
When I’m not frustrated, I feel sorry for doctors who are trying to treat something they don’t understand. I feel like Alicia in that I’ll probably never see a cure in my lifetime. And when a person gets older many doctors drop the ball with the idea of ‘at his age, why bother.’ Am I imagining this? Anybody else?
Without relying on what these AI engines report I would just throw all your positive results (and negative ones if you can) into them and see what they come up. My Perplexity report (I have the upgrade version) was fascinating and pretty much fit with what Dr. Ruhoy thinks is going on.
I don’t know about a cure but I would be SHOCKED if you didn’t find something that was a substantial help.
I did exactly that but ChatGPT3 o3 reasoning model.
It was able to tell me my symptoms to the core, explain what is wrong and offer potential treatments.
I would recommend also adding microbiome and nutrition tests in the mix as those were also related to my symptoms.
You are a superstar Cort. I will dig up my other tests and input into AI. Thanks so much 🙏
I may have chronic Lyme but no testing shows it. I was told CD8 #s aren’t specific to Lyme as are the rest of your test results. If IgeneX testing doesn’t show positive Lyme and you think you have it then I’d suggest following a protocol from Marty Ross MD here: https://www.treatlyme.net/. I’ve followed him for 9 years, using only non drug options, but he offers both. His recommendations helped me recover. That said there is probably no complete cure for Lyme without staying on the recommended herbs for a very long time, without a break. It took me 4 years to recover completely and now I still treat once a month and more in the summer when I could be bitten again. I use Cryptolepsis (Secret of the Tribes gylcerite) which is proven to kill all forms of Borrelia. If you aren’t familiar with the term herx, please review before taking and go low and slow. As to the Herpes, I have that as well. 90% of us do. We don’t have issues with it until our immune system are overwhelmed, exhausted by fighting all the time. Which is why it just starts ignoring the Lyme. And then it doesn’t show up on testing. You will find suggestions at the link above for treating Herpes as well. Many overlap with Lyme. 🙂 I use Olive leaf, monolaurin, cistus tea (https://botanicalinstitute.org/cistus/) to suppress my virus levels. It seems to be working as my symptoms are pretty much gone now so my body is getting back to normal. It’s taken 10 years and I still have histamine intolerance which popped up in 2020. I still take a lot of supplements but feel back to 90% normal. It is possible to recover! See my post above on genetic testing. It will speed things up in most cases. Blessings!
The fact is that after decades you only have hypotheses but unfortunately no effective treatment approaches…
I get the resignation, but we are making progress. We have better, more fully fleshed hypotheses backed by more data and—at least in this case—a potential pathway to understanding how this all got started – and when we understand that – the likelihood that immunomodulators will be available to help.
There’s real potential in that. Yes, it is dependent on funding – always a sticking point with us – but this group did get one large NIH grant and I would hope given their data that another is in its future. Until then non-profits like the PLRC and Solve ME (and lets hope Polybio) will keep them moving forward. Patients of course can help as well.
Looking at ME/CFS really objectively – with the amount of funding this disease has gotten – could we really have expected FDA approved treatments by now? Unfortunately no… For one thing, we won’t be able to get pharmaceutical companies interested in us until we have biological biomarkers – which is what this group thinks it can find – and on a very fine-tuned scale.
So, while yes, it’s been decades, way too long – no disease better than ME/CFS demonstrates how broken our medical research system is in some important ways – but I think that we’ve gotten to the place where the next five years or so will eclipse everything that’s been done in the last 20.
Time will tell and please hang on!
Why would the “system” want to find a cure for any disease…there’s far too much money to be lost on treatment and treatment drugs from the medical mafia.
Lawmakers have there fingers deeply embedded in the big pharma pie.
People being wisked away by force in the UK is enough red flags for me.
WORLDWIDE PUBLIC INQUIRY PLEASE!
This T-cell exhaustion theory of ME/CFS makes a lot of sense, because the high viral antibody titres seen in ME/CFS, and the persistent low-level enterovirus infections found in the body tissues of ME/CFS patients, suggests that something is blocking the immune system from clearing these viruses.
In this post, there is a list of supplement and drug treatments which might in theory counter T-cell exhaustion: https://forums.phoenixrising.me/threads/91606/post-2454922
Nice list! I like throwing in vasodilators and HBOT in there to increase oxygenation. What do you think about mitochondrial enhancers to buck up the T-cells energy production?
I was not aware of the mitochondrial connection to T-cell exhaustion, but doing a bit of web searching just now, I found a few studies indicating that mitochondrial energy dysfunction can increase T-cell exhaustion.
One study says: “defects in mitochondrial respiration are sufficient to elicit … T cell exhaustion even in the absence of continuous antigen exposure”. Source: https://pmc.ncbi.nlm.nih.gov/articles/PMC10611730/
And another study found that in HIV, exhausted T-cells can be energised again using mitochondrial antioxidant treatment and other mitochondrial manipulations: https://pmc.ncbi.nlm.nih.gov/articles/PMC9295450/
Very interesting – no antigen/pathogen required! Thanks for that. Their studies do, thus far, suggest an antigen is involved (but maybe not necessarily – in some cases it may just be the mitochondria! How about that!.
“At the molecular level, we find that mitochondrial dysfunction causes redox stress, which inhibits the proteasomal degradation of hypoxia-inducible factor 1α (HIF-1α) and promotes the transcriptional and metabolic reprogramming of Tpex cells into terminally exhausted T cells.”
And then there’s the T-cell exhaustion in HIV as well….
Very interesting.
I hope ME/CFS researchers are looking at other illnesses. Some of the most interesting papers I have read over recent months have related to fatigue in other conditions, such as autoimmune conditions
I second that regarding mitochondrial function Cort. The exhaustion on immunity cells could be related to impaired mitochondrial function.
If you look at both papers that HIP has identified they both mention PGC-1a which is required for mitochondrial biogenesis.
Both papers are very interesting, Thank you for bringing up the mitochondria connection
Efthymios…I’d like to know how you are doing now?
Have you had to tweak your tudca amounts or taurine amounts further?
I started on both a few months ago with good results but it seams what amounts I was taking I am having to up the amount to get the same results as far as stool color.
I’ve always read that a person’s stool color should be medium to darker brown or as TC Hale says, the color of cardboard.
Could be the heavy metal detox I’m doing that’s changing things I suppose
I am doing fine Thanks ! Just to give an update ,with the latest data and analysis I have been doing it seems that Phosphatidylcholine is crucial because of its cell membrane support abilities. Choline was a part of my regimen back then which also may be responsible for my remission. In your case something could be missing which has not been addressed yet.
Interested to know more about your experience with phosphatidylcholine, and how it helped.
A recent hypothesis pinpoints it as part of the issues in ME/CFS:
https://www.preprints.org/manuscript/202409.1467/v2
I quite like this hypothesis, but I do have a bias towards neurotransmitters playing a key role in the illness!
@Matthias, I am beginning to believe that my recovery can be attributed to a combination of Choline and Taurine and subsequent oxidative stress amelioration via certain antioxidants. My latest analysis using AI points to restoring the cell membrane first, and then improving peroxisomal and mitochondrial function. Thread here : https://x.com/lifeanalytics/status/1921926422932713970
Thanks! Might try taurine + choline + antioxidants for my daughter
HBOT is looking quite underwhelming in long covid studies
‘High viral antibody titres seen in ME/CFS’
Could you please show me studies that have confirmed this?
While there should have been much more research, still a lot of research has occurred over the past 30 years in ME/CFS, and nothing compelling has shown up in terms of viruses.
I consider that viruses trigger the illness, but do not perpetuate it.
People want a simple explanation, and I get it! Eg. Reactivated viruses cause ME/CFS, kill the viruses and….. ‘voila’! Cure!
I think this is extremely unlikely.
Let’s see if Polybio’s trials of antivirals have any success. I hope they do and I hope I am proven wrong. I would love it if my daughter could take a few pills each day and feel much better.
If the trials fail, I hope we can move on, once and for all, on the idea that viruses perpetuate the illness. But I doubt it. People will cling on to their beliefs, their theories.
Personally I think it is far more likely that a complex systemic issue perpetuates the illness. And I think the central issue lies in the brain / CNS / autonomic nervous system. Clearly, all these systems interact with the immune system, and there are all sorts of feedback loops.
Have a look at https://me-pedia.org/wiki/List_of_enterovirus_infection_studies which details dozens of studies finding enterovirus in ME/CFS patients. Also look at the brain biopsy studies finding enterovirus in the brains of ME/CFS patients: https://www.me-pedia.org/wiki/Autopsy_in_Myalgic_Encephalomyelitis
Further evidence of enterovirus involvement comes from the studies on interferon treatment of ME/CFS, which often dramatically improves enterovirus ME/CFS patients(but not herpesvirus ME/CFS patients). This suggests enterovirus is an active cause of ME/CFS. See: https://me-pedia.org/wiki/Interferon Unfortunately there are reasons why interferon is not viable as a long-term treatment.
But it is not just ME/CFS which may be caused by viruses and bacteria: one school of medical thought posits that most chronic diseases and cancers may be due to such microbes. This is because most chronic diseases have been linked to the presence of pathogenic microbes in the diseased tissues or organs. See this article: https://me-pedia.org/wiki/List_of_chronic_diseases_linked_to_infectious_pathogens Of course association does not prove causation, but there is a very real possibility that nearly all the diseases that afflict human beings result from pathogenic microbes.
Of course, even if microbes are causing most diseases, the causal mechanism may not be straightforward. In the case of type 1 diabetes, for example, which is linked to coxsackievirus B4 infection of the insulin-producing cells of the pancreas, many of these cells are not destroyed directly by the virus, but by the autoimmune attack against these cells that the virus triggers.
So if ME/CFS is caused by viruses, the mechanisms may be complex.
Also, the antiviral route to treating ME/CFS may not be the best option. There may be underlying immune issues which allow viruses to remain as long-term smouldering infections in ME/CFS patients, such as the T-cell exhaustion detailed in this blog article. In which case, the best treatment might be to rectify these immune issues, which may then automatically clear the infections.
I would have to look but in general it seems to me that antibody titers are kind of all over the place.
In my experience here and anywhere else today there isn’t a big group among patients who thinks that herpes reactivation is likely the pathomechanism of ME/CFS.
The most common reaction among patients to me suggesting that the cause of ME/CFS might be HHV-6b reactivation is disbelief. Their answer is that herpes can’t be the cause because if it were it would have been found out back in the 1980s.
I think the high viral antibodies may be a red herring in ME/CFS. I had no idea I had EBV/HHV6/Parvo etc., (likely from time in military in1980s) and had no health issues, was very active, before I gotten bitten by ticks and ended up with Lyme 2008 (maybe, can’t prove it) and Anaplasmosis (2015). It took 6 months to get treated with ABX for ANA, but I’m assuming my 102* fever for a week cleared it. I dealt with Lyme symptoms for 4 years before they stopped/ morphed into ME/CFS with severe fatigue/PEM/POTs. 2 years to recover from that to some extent. Two years after that, now,( 10 years total) and I still have the same high antibodies as tested in 2018 but my ME/CFS and other symptoms are pretty much gone. I still have MCAS which makes it hard to tell for sure at times. I’m due for a retest soon and will report back if I find my levels are “normal” but I don’t expect them to be. If they are I’ll stop taking the anti-viral herbs I’ve been taking for years and see if my symptoms come back and IgG levels rise again. But I agree with you, it’s way more complicated than this. Unfortunately.
T-cells clear viruses… Have a look at Jackie Cliffs research project about HHV-6b reactivation as the cause of ME/CFS.
https://cureme.lshtm.ac.uk/index.php/2025/03/10/first-cure-me-webinar/
This is interesting. I already know that I have an abnormally high number of
T-cells from previous testing. I’ve probably tried 100 or more various supplements and the only one that has helped me (besides LDN) is on that list. Huperzine-A helps my energy levels, and I originally tried it because it is close to Mestinon in terms of function, which also seems to have helped many of us.
I am with a health system that doesn’t really do much to assist with ME/CFS so I’ve pretty much been on my own.
Here’s another thing – T cell exhaustion does not exist early in the illness. It develops over time.
Yet we obviously have the symptoms early in the illness.
T Cell exhaustion is a consequence of the illness.
Actually I think it fits. You get the infection and either have trouble fighting it off – or sometimes the symptoms disappear entirely – then weeks later – T-cells exhausted (?) you’re in the soup. In that scenario T-cell exhaustion maintains the illness (?) The big question is what causes the T-cell exhaustion.
HHV-6 and HHV-7 are T-cell lymphotropic. And this is just one of many characteristics that makes HHV-6b reactivation so highly likely to be at the center of ME/CFS.
They are known to be able to slow down mitochondria to protect their replication. Infections start with T-cells but then jump over to neurons and inflame the brain typically in a “subacute” smoldering fashion just as we see it in ME/CFS.
Hornig et al found immune activation in the first few years of the illness before the immune system becomes exhausted.
If this is correct, there is absolutely no logic to suggest immune exhaustion causes symptomology in ME/CFS ( but it might eventually help perpetuate symptomology – see below) Because the symptoms exist early in the illness, when there isn’t immune exhaustion!!!
I don’t think Selin et al’s study looked at the temporal dimension?
While I am not convinced by viral reactivation being central to the illness, I concede it is still possible. There might be the following chain of events:
1. Illness trigger (virus)
2. Trigger reactivates virus/s
3. Symptoms generated through multiple systems, including immune system activation
4. Immune system (T cells) eventually exhausted
5. T cell exhaustion helps to perpetuate the illness, as it becomes even harder to clear virus/s
If this is correct, then yes things that treated the t cell exhaustion, as well as antivirals, might help. But reactivation of viruses is central, and causative.
Antivirals have failed repeatedly. Polybio are trialling a couple, including Truvada which is effective against a number of viruses.
Let’s see how the trials go. As I said, I really hope they are successful. Swallowing a few pills every day to get significant improvements would be amazing!
I have looked into two antivirals studies more closely: One was about acyclovir. A study from the 1980s. Another was published just a couple of months ago and different drugs were tested.
In my view both studies were useless because they didn’t differentiate acute and non-acute phase of ME. It is well documented that it is only in the first years that patients have ongoing acute inflammation episodes.
Later because of pacing acute episodes become rare and short lived. But people don’t recover anymore or only extremely slowly because organ and tissue damage in the mitochondria, the brain ect. is irreversible.
The problems with the ME/CFS antivirals studies is that they don’t take into account that people with ME/CFS can be extremely disabled while it is rare that they have flare-ups or even extended inflammatory episodes.
What can we expect of an antiviral? An antiviral may suppress an acute infection with a virus. Can we expect that it cures the damage that years of ME/CFS inflammation did to the body? No.
In my view all the antiviral studies haven’t taken this into account. So what they really do is that they test whether ME/CFS organ damage can be cured with an antiviral. But this is trivial. It will of course not work. Antivirals can’t do that. And we don’t need studies to prove it.
But there isn’t compelling evidence at all of consistent organ damage.
A massive study of thousands of ME/CFS patients from the University of Edinburgh, in preprint, doesn’t show anything like that.
The only thing it shows is a few things like mildly abnormal liver function and if I recall some mild insulin issues.
I am sure some patients have organ damage, but it’s not something the science shows is common or consistent.
My view is the antivirals haven’t worked because either they aren’t effective enough, or viral reactivation really isn’t a thing.
Polybio are testing Truvada which has shown strong effectiveness against several viruses. I hope it’s successful.
If it isn’t I guess there will continue to be patients who cling on to the virus reactivation theory .
Again, as far as my experiences go, it is really not the patients who think virus reactivation is a thing.
The question whether it is causative for ME/CFS is back because of new and promising research results who were attained in the tens and could be substantiated over the past years by research teams from all over the world.
The idea got a further boost because there were reports that early Long Covid patients benefitted form herpes drugs and because several papers who dug into the question of the cause of Long Covid came up stating that there was a connection to herpes.
It is not the patients but it is researchers like Iwasaki and Klimas, and Selin, Gil and Kumar whose work is discussed here who think that viral and herpes reactivation is a sound hypothesis to explain ME/CFS pathomechanism and should be researched further.
Regarding the antivirals studies I can only repeat what I said above. As long as the researchers don’t understand the remitting and relapsing nature of ME/CFS these studies will only bring negative or contradictory results by design.
I find it difficult to understand why you claim that there is no organ damage in ME/CFS under a post where Cort has explained to us that a team of immunologists can now describe the pathological changes in the T-cells in ME/CFS.
Over the past four decades there has been an abundance of studies that have proved repeatedly and in studies independent of each other that there are pathological changes in the brain, the vessels, red blood cells, mitochondrial function, neural signalling, and last but not least the immune system.
Here is a resarch overview
https://www.mecfs.de/was-ist-me-cfs/pathophysiologie/
on the subject.
My EBV, HHV6, Parvo have never tested as being reactivated (7 tests).
This may confirm our suspicions that Reactivation is not necessary for the virus to be an issue: https://www.healthrising.org/blog/2024/08/05/herpesviruses-blood-flows-chronic-fatigue-long-covid/. “While doctors and researchers commonly look for signs of viral reactivation, establishing latency may be the more important factor when it comes to long-lived chronic diseases like ME/CFS, FM, and long COVID”. So maybe another illness or injury damages cells, releasing the latent virus, which the immune system then recognizes as a threat. So now the body is fighting 2 illness and immune system is exhausted.
Or, my current theory about the role of viruses, that even after, or without, an illness trigger, just the usual daily death of some cells, that now have latent viruses in them, keeps the immune system on high alert all the time leading to exhaustion. That would explain my high levels of antibodies on testing without reactivation. I take anti-inflammatory herbs daily which are keeping inflammation and the immune system tamped down and that allowed it to eventually get back to a semblance of normal. My immune system seems to work ok for other “bugs” and after a few years of Covid vaccines seems to react appropriately now to new vaccines, like flu & shingles, without the past overreactions. . The mast cells are still fired up about histamine though. Latent virus in cells are not effected by antibiotics, just like the round body/cyst intra-cellular form of Lyme can’t be killed which would explain why antivirals fail, if the viruses are not reactivated but latent.
‘Weeks later’? Do we know the T cell exhaustion occurs that early?
The big study by Hornig et al, from 2015, showed immune activation for the first few years of the illness, before eventual under activity/ exhaustion
That matches my history, 3-4 to full blown ME/CFS/ severe fatigue.
Thank you for this update, Cort. I am glad, that there is more and more research on T-cells.
HHV-6b is T-cell lymphotropic.
As a patient who responds very well to the herpes drug acyclovir during an acute ME/CFS inflammation I am glad that it seems that these researchers are aware that it might not be autoimmunity but simply a herpes virus that causes the changes that they observe.
Are Selin and Gil also actively investigating that connection?
Jackie Cliff and Eliana Lacerda who research HHV-6b as the cause of ME/CFS in a project in London at Brunel University also investigate T-cells.
Here they gave an update on their projects in early spring:
https://cureme.lshtm.ac.uk/index.php/2025/03/10/first-cure-me-webinar/
I would love for you to cover their research on HHV-6b and T-cells in a follow-up to the work of Selin and her colleagues, Cort.
That’s interesting, Lina. Prusty has also done interesting work in this space.
I think the HHV6 theory is plausible, for sure, even if I don’t think it’s the most likely explanation. I guess we need to wait and see what the research shows.
I agree it would be nice to have an article on the latest progress on HHV6 and me/cfs.
In 1984, I was diagnosed with immune deficiency and chronic encephalopathy by nationally recognized immunologist, Dr. Sol Klotz and his research partner from the University of Central Florida, professor of immunology, Dr. James Sweeney.
I had only 372 T cells and a complete reversal of T4/T8. Although this was trending toward a profile of AIDS, I did not have HIV.
The doctors immediately put me on an anticoagulant that was a orphan drug from Germany. I was also given shots of transfer factor.
It always seemed to me that they knew exactly what I had.
When they retired, I became a patient of Dr. Paul Cheney who did extensive testing at research laboratories. I was positive for HHV-6 A which is nothing like HHV-6 B. HHV-6A is a factor in the progression of ARC to AIDS and has also been associated with the development of MS.
I am convinced that most ME/CFS patients have never had the battery of immune tests I had more than 40 years ago, nor have they been tested for HHV-6 A.
Yes, T cells are exhausted. Yes, activity can be followed by PEM. We knew this a long time ago.
New investigations have suggested something in the blood or stomach is driving ME/CFS. Why haven’t we found out what that is instead of reinventing the wheel and asking for more funding to continue in the same kind of research?
If it is something in the blood, it makes sense that diluting whatever it is with an anticoagulant would be useful.
I just watched Liisa Selin’s presentation that is embedded in the post. I found it very interesting that she suggested that it was theoretically possible that different subgroups of patients had reactivations of different viruses. Also, I found it great that she presented a complete list of known viruses that could do the job.
I am a believer in Jackie Cliff’s and colleagues HHV-6b theory of causation of ME/CFS. I find it interesting that you were tested positive on HHV-6a back in the days.
It’s true. These hypotheses were around already 20-30 years ago. Maybe the 1990ies backlash to liberalism has killed this research?
Now all these projects are again threatened by the political change.
I am really happy that researchers in the US and in Britain think that autoimmunity or viral reactivation are a possibility. The leading German researchers have given up on pathogens and have already convinced themselves of autoimmunity as the cause it seems.
OK, after many years of reading research findings, I’m finally intrigued. I was told back in the mid 90’s that I have a huge overabundance of T-cells, but nobody was able to tell me what this meant from a clinical standpoint other than my immune system is overactive.
I’ve never had any other findings that potentially relate to my ME/CFS, but I’ve always assumed that this somehow holds the key, since my fatigue is very much like “sickness behavior” which would be expected when one’s immune system is ratcheted up as though you are always sick.
That said, I don’t know if these T-cells are at all like the ones discovered in this study, but it makes me wish I had the connections to find out.
I enjoyed this webinar and this is a great breakdown of it.
“Ultimately, these researchers want to uncover biomarkers corresponding to the different molecular subsets and produce tests that doctors can use in the clinic.”
I would really like to see the Selin Lab create a page for their Biomarker ambitions with a plan and a link for specific Biomarker/Diagnostic test fundraising.
The fascinating paper in the link below shows that there are reasons beyond viruses as to why T cells might get exhausted. I think B adrenergic signalling is a fascinating possibility, given it impacts on so many systems in the body – as ME/CFS does:
https://www.nature.com/articles/s41586-023-06568-6
Very interesting. Somewhere in the nervous system lies the cause . Catacholaminas.
Fantastic write up and fantastic research.
It is clear to me that the immune stimulus acts as a trigger, and that once the die is cast a cycle of dysfunction persists that is very difficult to recover from.
My only gripe is that “immune stimulus (infection)” is not always the case. I developed ME following a vaccine, started after the first and got worse after the second dose. ME forums have many others in the same boat. “Immune stimulus” would be perfectly accurate if you don’t want to explicitly mention those of us here as a result of a vaccine. But, we need to be included. The very fact we exist should help guide researchers towards an answer that helps everyone. I appreciate the sensitivity of discussing it, but failing to discuss it leave a not insignificant cohort of patients with very little acknowledgement, which as many with ME know is harmful in itself.
I think it’s pretty clear that vaccines can trigger these diseases and I would be surprised if these researchers didn’t include them in the list of initial immune triggers. Thanks for the reminder though. 🙂
https://www.healthrising.org/blog/2024/01/22/vaccines-long-covid-protectors-exacerbators/
Is there a study that has made a distinction with the duration of the disease? NK-T cell exhaustion patients who have been ill for less than 3 years and patients who have been ill for more than 3 years. And is there a difference between patients with a bacterial and viral infection as the onset? Or patients where it developed after a stressful event. This NK-T cell dysfunction was already found by Dr. D. Peterson 40 years ago, I think. And also by Professor Klimas. Nothing new under the sun. Correct me if I am wrong.
Nobody claims that this is a new finding. What is new is the depth of the understanding of T-cell pathology.
The Cure ME team from London led by Jacqueline Cliff researches T-cells too and they make different distinctions. They distiguish between a mild/moderate and a severe ME group and they also have a Long Covid group with ME symptoms.
The important distinction that they have brought into ME/CFS research is that they consider the remitting and relapsing nature and fluctuating symptom severity to be an important thing when researching the immune system and therefore design studies where they can track this over time.
Here’s a presentation of their findings and ongoing projects from Feb 2025.
https://cureme.lshtm.ac.uk/index.php/2025/03/10/first-cure-me-webinar/
Thx Lina for your information 🙂 First of all, I don’t think that this exhaustion is the cause of ME, but rather a consequence of something else (in the loop). Additionally, it is alarming because then the risk of cancer is significantly increased in ME patients.The cause is not located in the immune system but in the nervous system (stress system), at least that is my belief. (that gives an increased change of heart failure). Both we see in the literature.
Yes I definitely think the main issue lies in the CNS + autonomic nervous system. But these clearly intersect with the immune system. It’s just that, in my opinion, immune system involvement is secondary.
Many patients report benefits with ADHD medications.
Matthias, you imply that a pathogen like the flu, EBV or Covid could go to the brain, trigger ME and then in the course of the illness causes pathology in the immune system.
Where’s the logic?
Here’s the logic . Feel free to critique.
1. Initial trigger – viral, bacterial, stress or combination
2. Immune system activation and dysregulation of CNS / ANS
3. Initial trigger cleared, but chronic problems remain in the CNS / ANS that drive symptomology
5. Dysregulated neurotransmitters Drive mental fatigue as well as PEM (Systrom’s theory starts with the CNS)
4. Immune activation eventually becomes immune exhaustion (as per Hornig et al’s large 2015 study). This process is driven by the ANS issues, rather than a chronic virus or reactivated viruses
Sorry I typed quickly and the numbers are out of whack
My pleasure.
I don’t think that you claimed that the exhaustion is the cause of ME. I think everyone here thinks that the researchers now have to find the cause for this.
In my view HHV-6b is a perfect fit for these findings. : )
Since I was a mild patient for several years I think that your idea that ME begins in the nervous system is wrong. For several years my symptoms were flu-like, were triggered by exertion and went away when I rested a couple of days.
I was only when these inflammatory episodes got more intense and out of my control that I also began to have brain inflammation and the typical cognitive symptoms.
This and my good responsitivity to acyclovir makes me think that Cliff and others are on the right track with their HHV-6b research.
It was you that first tought me that HHV-6b first infects T-cells and later neurons by the way.
In the clinical consensus statement on ME/CFS for the German speaking countries it is mentioned that ME patients have increased cancer risk, especially lymph cancers. But there are of course not heaps of data that cover that ; )
Having ME and then getting cancer maybe isn’t as bad actually. You’d experience an instant upgrade from being a patient with a scapegoat diagnosis to becoming a patient in one of the best funded and always advancing medical fields. Cancer patients even have their specially trained psychotherapists when we’re nowhere near to having enough doctors. ; )
Comment *in the medical field?
It is interesting that Maureen Hanson has taken similar position over time as Dr Chia that enterovirus infections may be the driver of main subset of MECFS patients. Non cytolytic form of virus that can not be completely cleared but that chronically stimulates T cells and causes mitochondrial dysfunction. I recently read researchers studying CRISPR/Cas13 can be used as broad antiviral as well as potential method to diagnose whether cells are infected with ssRNA. Cort – can you reach out the Dr Hansen and ask whether she has considered this new method. This deserves further study and funding.
https://pmc.ncbi.nlm.nih.gov/articles/PMC7985958/
https://www.genengnews.com/gen-edge/stepping-on-the-cas13-carver-biosciences-uses-crispr-to-target-rna-viruses/
I am very interesting in learning whether anyone in this forum has tried Metformin for ME/CFS or Long Covid. If so, what was the outcome? There are studies show a greatly reduced incidence of LC when Metformin was taken early in the infection, but I haven’t found anything on treatment of LC that has already occurred.
Metformin has some important side effects so taking it would have to be worth any risk.
According to Professor Humbert, an internist and dermatologist, author of several books on parasites, worms such as helminths, ascarids, pinworms, etc., induce immunosuppression that favors infections related to the herpes virus, shingles, Epstein-Barr virus, lymphomas, digestive cancers, breast cancer, cervical cancer, Parkinson’s, Alzheimer’s, eczema, psoriasis, and more. He hypothesizes that the disappearance of parasites could help restore the immune system to aid in eliminating COVID-19 and many other diseases. If there is an allergic condition or various intolerances, and removing all dairy products and gluten is not enough, it is likely that parasites are present.
The problem with parasites is that they are not always detectable. Some worms reside in the liver, others in the intestines or spleen, and some form cysts. But the good news is that there are medications to eradicate parasites.
I read an article yesterday about autopsies performed on patients with multiple sclerosis, and 100% had harmful helminths in their bodies.
Traduction Chat GPT
I took one single dose of ivermectin…I became itchy all over and about a week later I had much sustained improvement in my over all health. Several subsequent doses have had zero effect
The treatments will vary depending on the parasites present in the body. For some worms, it will be Albendazole (which also induces the death of cancer cells in breast cancer), while for other worms like Ascaris, it will be Mebendazole (which also acts on malignant cells in melanomas and glioblastomas)… and sometimes, multiple antiparasitic medications are needed for a synergistic effect.
It’s very complicated, and to my knowledge, no EM/SFC or long COVID experts make the connection between these diseases and parasites. But is there a link?
Yes, ive also had some smaller improvement with fenbendozole.
Ask a dr about parasites…they say they don’t exist which is out right halarious
I have been following some alternative drs and tried to follow a protocol that brings parasites to the surface and kills them. I’m told I didn’t hit it hard enough & they told me a got a mast cell activation for it. Since then I have had big problems with sleep, nerve pain and gut. There is a protocol to treat parasites that is old and apparently works but you have to do the full thing or could make yourself worse: themilkcleanse.com. It’s 8 days of drinking cow, goat or other animal milkevery 2 hrs with herbs that kill parasites and no other food because parasites love milk. Anyhow my understanding is most everyone has some level of parasites but those who are sick may have a whole different level. I have also learned that it is very hard to find them on a lab because they decintegrate quickly out of the body. I was so sick my doc had me use Low dose immunotherapy with Dr Ty Vincent for lyme , yeast, parasites and that curtailed the gut part but didn’t help the nerve pain that was triggered. I hope to get stable enough to actually address it but i am 75 and have had me/cfs for 35 yrs and have become over sensitive to so much that might be helpful.
Thank you to the researchers for their hard work, and the organisations and patients that supported their research getting started.
And thank you for another great summary, Cort.
Polybio leading the way again with this good and important study:
https://polybio.org/polybio-and-weme-supported-study-identifies-two-distinct-me-cfs-subtypes/
In 1986. two researchers (Ablasshi and Salahuddin) working in the Gallo AIDS lab discovered a new virus. It was so lytic (ability to kill cells that they had trouble keeping it alive long enough to study it. When they were finally able to do this, they found it was killing B cells so they named it Human B Lymphotropic Virus (HBLV).
Then, they discovered it was also killing T cells so they renamed it HHV6. Following that, for reasons that were never explained) they subdivided this into HHV6-A and HHV6B.
These two herpes family viruses are not to be confused with each other. HHV6-B is ubiquitous in the population and the cause of childhood roseola.
Not so, with HHV6-A. We have no idea of the prevalence since you can only be tested through research labs.
If you want to know about all the most recent research on HHV6-A and B, go to https://hhv-6foundation.org/ (Think about why there is a foundation dedicated to studying just these two viruses.)
Once you are infected with a herpes virus whether it is EBV, CMV, the chickenpox virus or HHV6 A and/or B, it never goes away. These viruses live in you body forever and may cause no problems unless something lowers immunity allowing them to re-activate. Extreme stress, surgery, toxic exposures?
The famous “bubble boy” was killed by EBV when he received a bone marrow transplant from his sister who had EBV as a young child. He had no immunity to control the virus so it caused cancerous tumors all over his body.
The key to all this is a kind of “chicken or egg” equation. Does the virus lower immunity allowing all these other viruses, bacteria and mycoplasma to proliferate or does some immune insult allow the virus to reactivate?
Since there is no cure for any herpes virus, it appears that increasing immunity so the body does its job is the best course of action.
Great article, Cort, thank you for putting this together. These researchers really seem to be getting at the core immunological alterations/dysfunctions. And once they do, there are (as you note) many available immuno-modulators, some of which hopefully will be effective for specific subsets of patients. It’s too bad that randomly trying these agents out before more is known would be a bad idea for us patients (due to them possibly amplifying the dysfunction rather than addressing it, not to mention their toxicity).
Right! Dr. Klimas has felt the same way. She wants to know which patients to try these strong drugs in. Once we can those subsets though….It just seems so enticing. I sincerely hope researchers are gloaming onto the possibilities. I just saw something similar is underway (I think – if I understand it!) in long COVID.
Does it have to be ‘strong drugs’? Klimas and Selin were involved in a small study on a nebulized antioxidant agent that seemed to show real promise in addressing symptomology as well as immune markers.
It was a very small study but hinted at real promise.
Given that, is further research occurring on this agent? If not, why not? Funding?
Was there something faulty with the study?
It would be nice to know. It’s a really annoying tease when researchers do these studies and get these impressive results, then nothing happens…
No it doesn’t have to be a strong drug at all does it? That nebulized antioxidant actually moved the needle on the T-cells. I don’t know about the research but I would be shocked if it was not continuing.
In the first few months of Covid a friend sent me an article. It said it wasn’t in a viruses interest to kill its host. That Covid and humans would eventually adapt to ‘living together’ and Covid would become more like a nasty cold. It made me think of ME. It is in a viruses interest to disrupt and debilitate its hosts immune system so the virus has more time to replicate and infect others. Also as the virus mutates it can come back and reinfect the compromised host more easily. Maybe why ME happens?
I’ve learnt over the years that ME researchers have to believe in their theory 110% to be able to pursue it hard. So now just take a passing interest until they produce more conclusive evidence, but I really do hope these researchers are onto something and can finally make some true progress with this dreadful illness.
Precision Life – an Oxford company, have identified 2 subsets in the DecodeME ME samples. One group has mutations in a gene involved in recharging their cells energy supplies after they are depleted. Another group has mutations in genes involved in production of neurotransmitters. The 2 ME groups have different symptoms. It’s obviously more complex than that, but they too are hoping to find suitable treatments tailored to help each group.
I agree that identifying subsets within ME is the only way forward as far as treatments are concerned.
Sure hope Dr. Bruce Patterson’s group and other’s are plugged into this research. The plot is indeed thickening – and it seems like all this has to coalesce soon! Thanks Cort!
RFK Jr.’s son has Long Covid and he wants research done on a cure. Contact him. Maybe he can get this going!